Choosing a mammalian cell line or other type of host is as critical a question as any other in the complex world of monoclonal antibody (mAb) manufacture. We take a look at the present status, with fed batch Chinese Hamster Ovary (CHO) cell culture in a dominant position, and reflect on the ever-present need to keep upstream and downstream development teams talking to each other.
Following the first authorized use for a biotherapeutic in 1987 (Genentech, ActivaseTM/tissue-plasminogen activator), Chinese Hamster Ovary (CHO) cells have become the dominant host cell for manufacturing complex biological molecules, in particular monoclonal antibodies (mAbs). Whether due to an innate property of the cell line or the dedication of cell biologists and bioengineers (probably all of these), mAb product titers have increased enormously over the years. Today, a figure above 10 g/L is not uncommon. The platform technology approach also tends to resist big changes, such as a switch to another cell line. “Don’t fix something that isn’t broken” is an old maxim much loved by heads of production. By the end of 2018, over 60% of approved mAbs were produced in a CHO cell, system.[1]
Distribution of host cells used for mAb production in 2019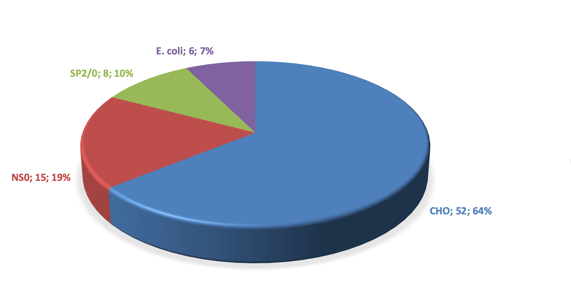
This successful focus on increasing mAb titers has led to an evolution in the challenges that need to be addressed by the rest of the production process. For various reasons, high titers might be associated with more host cell protein impurities or higher levels of host cell DNA contamination. Changes in upstream processing might encourage the formation of dimers and aggregates, or lead to high concentrations of proteases. Perhaps the rapid rate of protein synthesis results in imperfect glycosylation. Even simple changes in additives or conditions during cell culture can impact other process steps. A healthy outcome of this situation is that upstream process experts and those focusing on downstream purification need to match their efforts, instead of one team blindly focusing on titer and the other desperately trying to increase capacity whilst maintaining purity and yield – a classic dilemma for protein purifiers.
 Manufacturers of protein A resins and other chromatography media for mAb purification need to observe and keep abreast of developments. The high titers push us to address dynamic binding capacity. Challenges with host cell proteins and DNA impurities can be approached by the novel use of a pre-column designed to protect the protein A column.
Manufacturers of protein A resins and other chromatography media for mAb purification need to observe and keep abreast of developments. The high titers push us to address dynamic binding capacity. Challenges with host cell proteins and DNA impurities can be approached by the novel use of a pre-column designed to protect the protein A column.
Will CHO cells in fed-batch reactors continue to dominate mAb production? That appears likely, but we’re keeping an eye on it. Upstream surprises, no matter how positive they might sound, are not good for a downstream processor.
Read more: Three market changes that will impact the production of mAbs and Are mAbs the ultimate targeted therapy? mAb purification - an overview of the essentials
Reference
1. see FDA drug labels, e.g. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm592464.htm
All product and company names marked with ™ are trademarks or registered trademarks of Bio-Works or of the respective trademark holders. Use by Bio-Works of trademarks of other holders does not imply any affiliation with or endorsement by them.