Introduction
Solid-phase synthesis of oligonucleotides generally gives material of rather high purity. However, for many applications, and especially for therapeutics, there is a need for purification to remove incomplete or erroneous sequences. Anion exchange chromatography (AIEX) is an efficient technique for oligonucleotide purification giving high purity and good yield in a single step, but optimization of the binding and elution conditions is needed. When the process conditions giving required purity and yield have been developed, scale-up can start. We have investigated the change in the purity vs yield correlation when transitioning from low sample load to high sample load.
Purification Optimization
The resolution of oligonucleotide purifications on WorkBeads™ 40Q was compared for NaCl-elution in the presence of 20 mM Tris-HCl, pH 8 and 20 mM NaOH, pH 12 (Fig. 1). The main peak was collected in 1-mL fractions. Each individual fraction was analyzed for yield and purity on a DNAPac PA200 analytical IEX column (Thermo Fisher Scientific). The WorkBeads 40Q resin was also compared to Capto™ Q ImpRes resin (GE Healthcare). The dynamic binding capacity (DBC) was determined by frontal analysis at 150 cm/h to 48 mg/mL resin by applying the crude oligonucleotide preparation from the solid support without further adjustment of the feed.
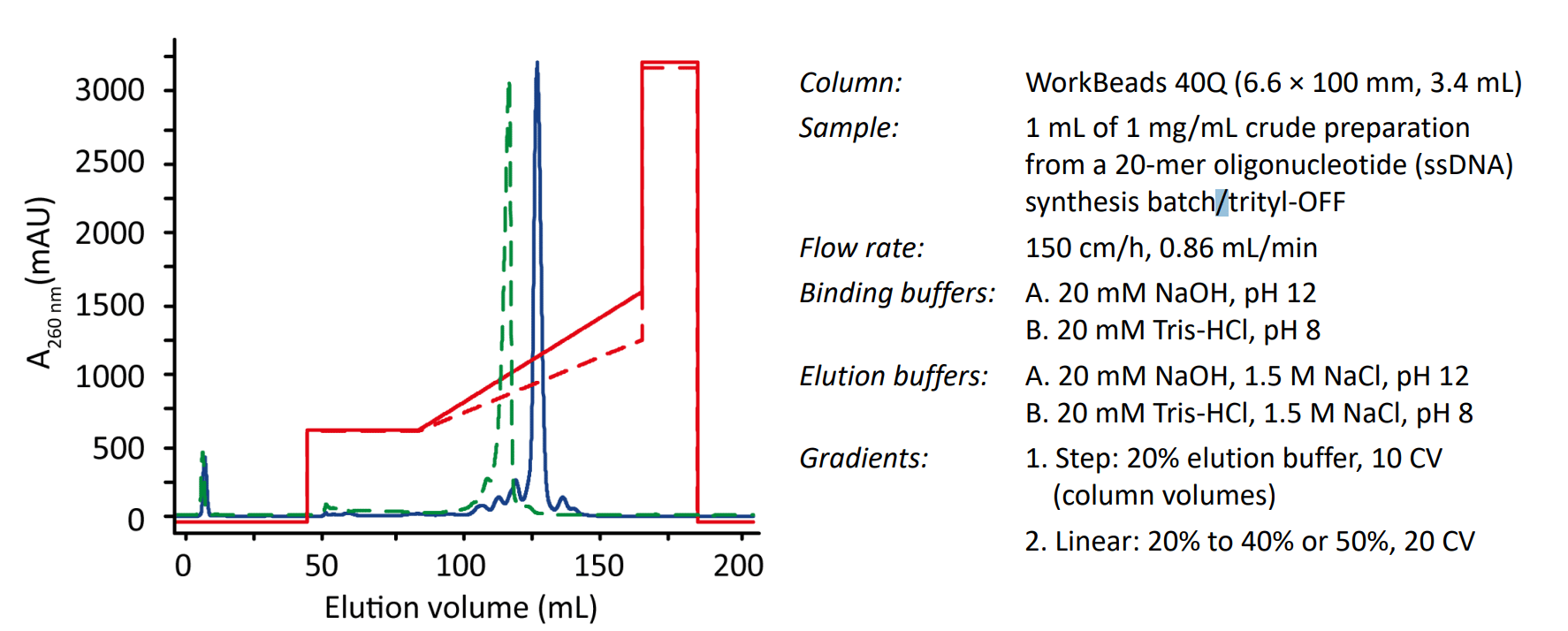
Figure 1. Purification of oligonucleotides on WorkBeads 40Q using 20 mM NaOH, pH 12 (solid blue) or 20 mM Tris-HCl, pH 8 (dotted green). The elution gradients are shown in red (dotted line 20-40% for 20 mM Tris-HCl, pH 8, and solid line 20-50% for 20 mM NaOH, pH 12).
Purity and Yield
For optimization, selected 1-mL fractions were combined to assess the effect of pooling on yield and purity, see Fig. 2 and Table 1. No significant difference was seen for the two buffer systems used for this oligonucleotide batch. A purity of 95.6% with 74.2% yield was obtained with the broadest pooling using Tris-buffer (Fig. 2B). Higher purities could be obtained by a more narrow pooling, as illustrated in Figure 2B. WorkBeads 40Q gave both higher purity and increased yield compared to Capto Q ImpRes (Table 1).
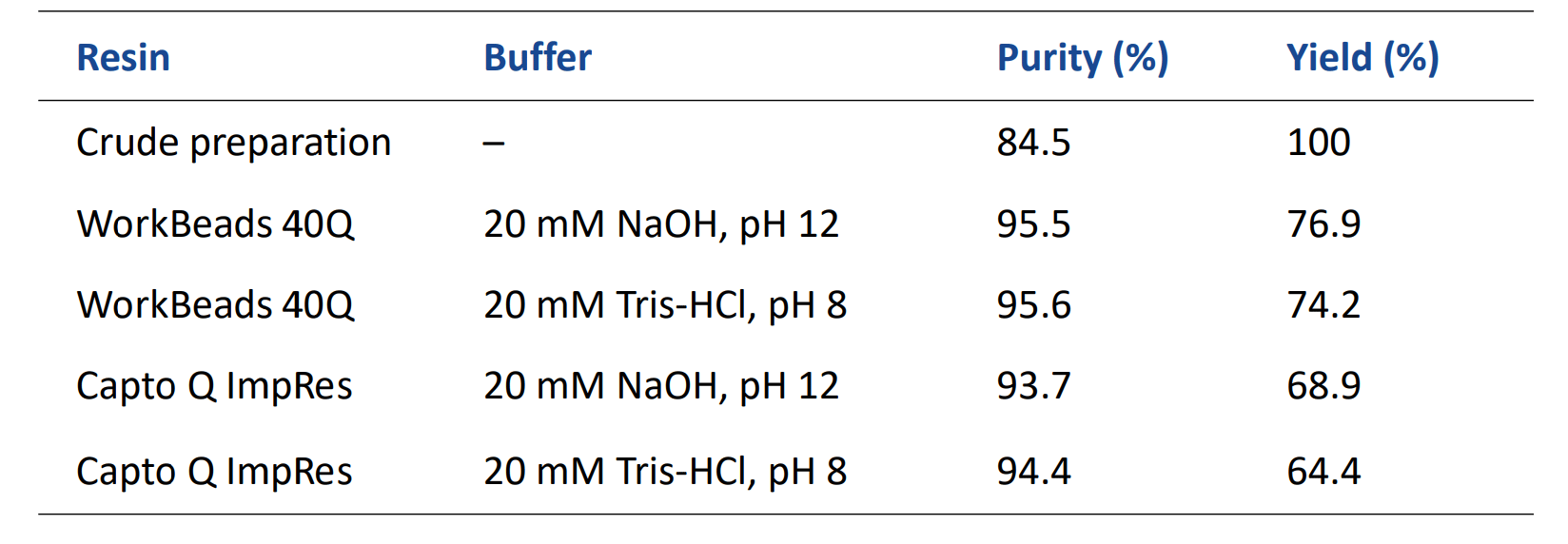
Table 1. Yield and purity. Pools of seven 1 mL fractions of the main peak were compared.
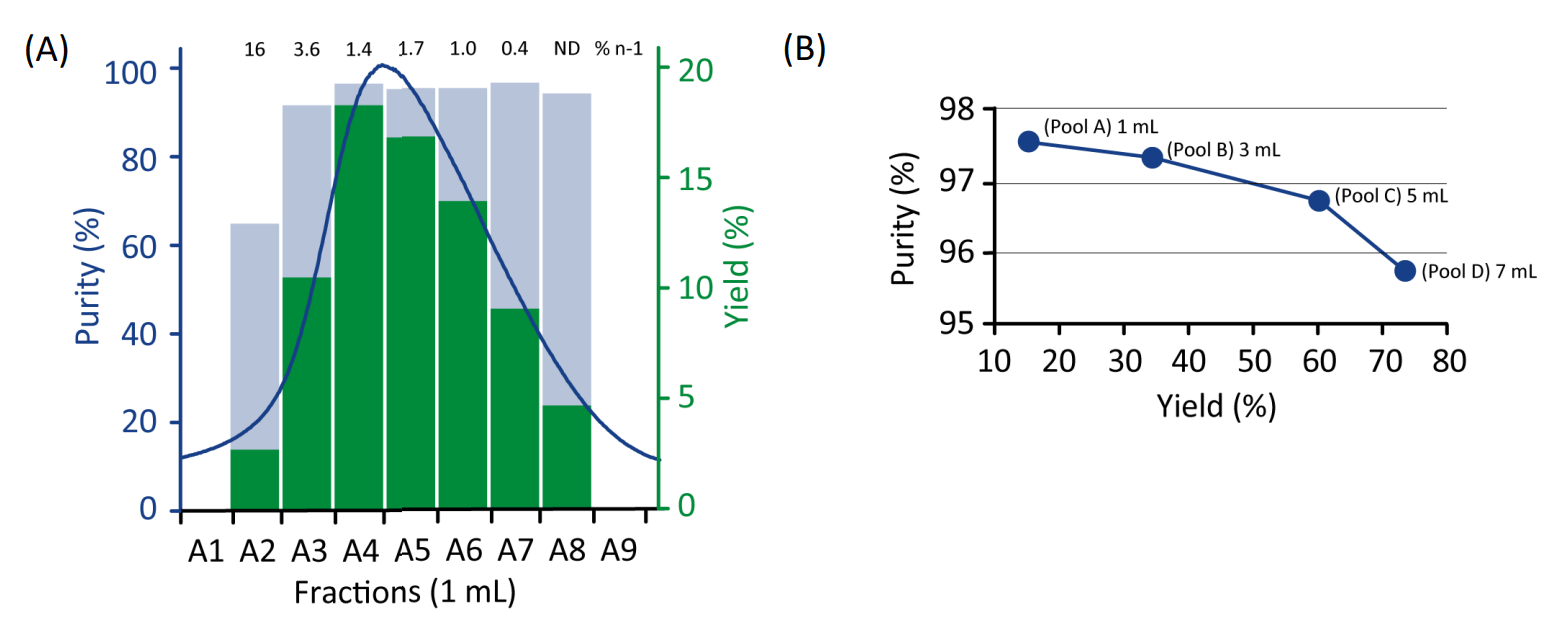
Figure 2. (A) Purity (light blue bars) vs yield (green bars) analyzed over the main peak. (B) Purity vs yield plot for different fraction pools (1 mL, 3 mL, 5 mL and 7 mL) of the eluted main peak obtained with 20 mM Tris-HCl, pH 8.
Scale-up
When the process conditions giving required purity and yield have been obtained, scale-up can be done. To investigate scale-up conditions, sample load of 80% of the resins DBC, i.e., 132 mg of the oligonucleotide preparation was loaded to the column.
Figure 3A shows a typical recovery shape of the full-length oligonucleotide for a process purification run with a sample load corresponding to 80% of DBC. In the beginning of the elution gradient the N-x species are eluted, and the full-length oligonucleotide starts to elute later in the gradient. The histogram in the chromatogram in Figure 3B shows the purity in individual fractions. This demonstrates the separation of the full-length oligonucleotide.
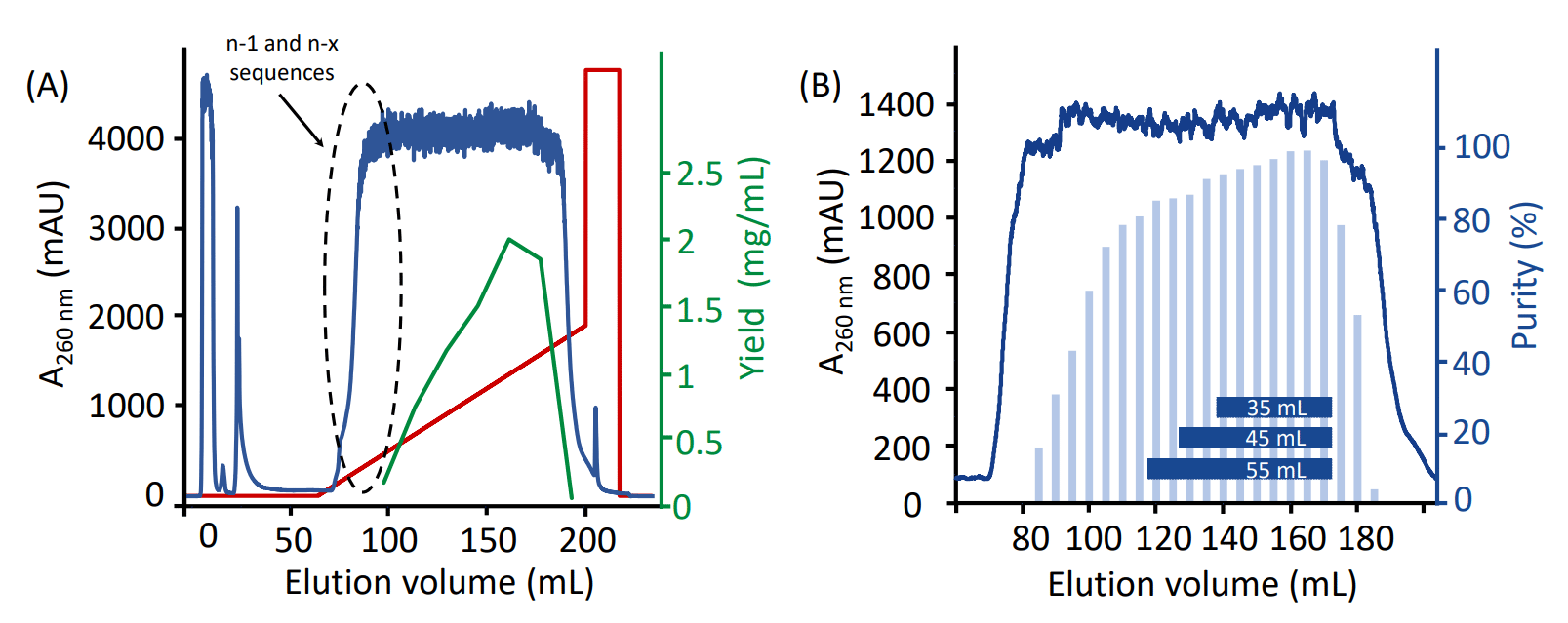
Figure 3. (A) Eluted oligonucleotide peak after loading an amount of crude oligonucleotide corresponding to 80% of the DBC. Green line represents full-length yield measured in each fraction and dotted black line visualizes presence of n-1 and n-x species. (B) Purity of full-length oligonucleotide (light blue bars) was measured in individual fractions. Pools of collected fractions are visualized (as horizontal bars) in the chromatogram.
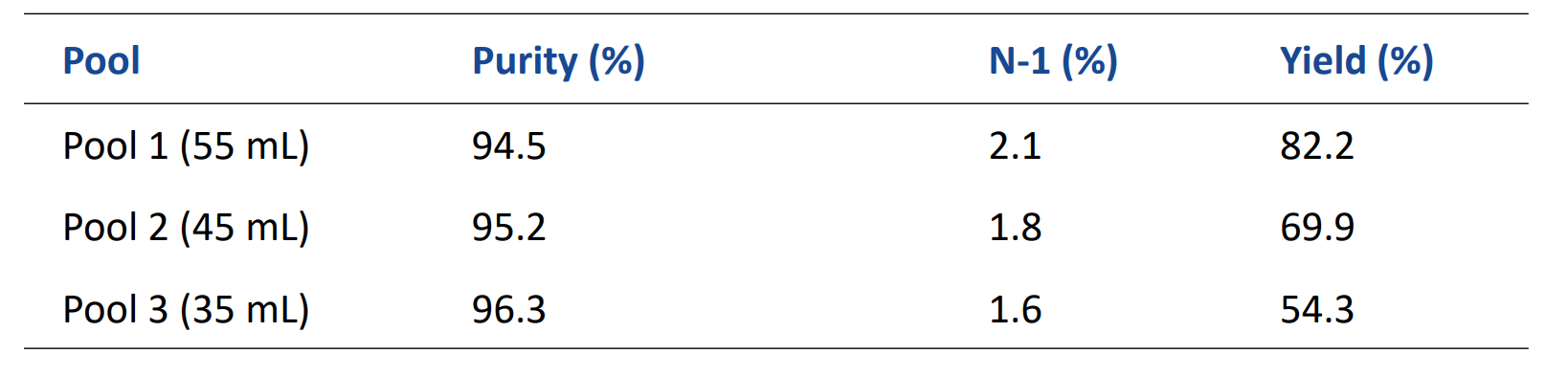
Table 2. Effect on yield and purity using different pooling after the 80% DBC run.
Conclusions
Purification of a 20-mer oligonucleotide using WorkBeads 40Q gave excellent purity with good yield in both NaOH- and Tris-based buffers. Both purity and yield were higher on WorkBeads 40Q compared to Capto Q ImpRes. The rigidity of WorkBeads 40Q (45-µm bead resin), allows for efficient purifications of full-length oligonucleotides also at process scales.