Introduction
RNA molecules have emerged as important molecules in therapeutics, primarily in the form of antisense oligos (ASO) and small interfering RNAs (siRNAs). Consequentially, requirements for their purity are very stringent. This level of purity is often achieved by high resolution anion exchange chromatography (AIEX) which can separate full-length sequences from incomplete or erroneous sequences by virtue of the negatively charged phosphate backbones.
RNA: 45 nts, L-RNA
L-RNA (L-stereoisomers)
• Good to use as therapeutics
• High biological stability
• Can escape the innate immune surveillance system
Binding optimization
Since RNA is less stable at basic pH, a neutral pH was maintained under RNase-free conditions to avoid decay. Three different resins were included in this comparison in which temperatures and residence times were investigated (Figures 1A-B). The dynamic binding capacity (QB10%; DBC) was determined using frontal analysis by applying the RNA preparation (solid-phase synthesized) under optimized conditions described below. The KD value is a measurement of the pore volume that is accessible to the molecule (Figure 1C).
The DBC measurements clearly demonstrated that WorkBeads 40Q is the best option for obtaining a high loading capacity (56.4 mg/mL; 1410 OD/mL) for this 45 nt-long RNA.
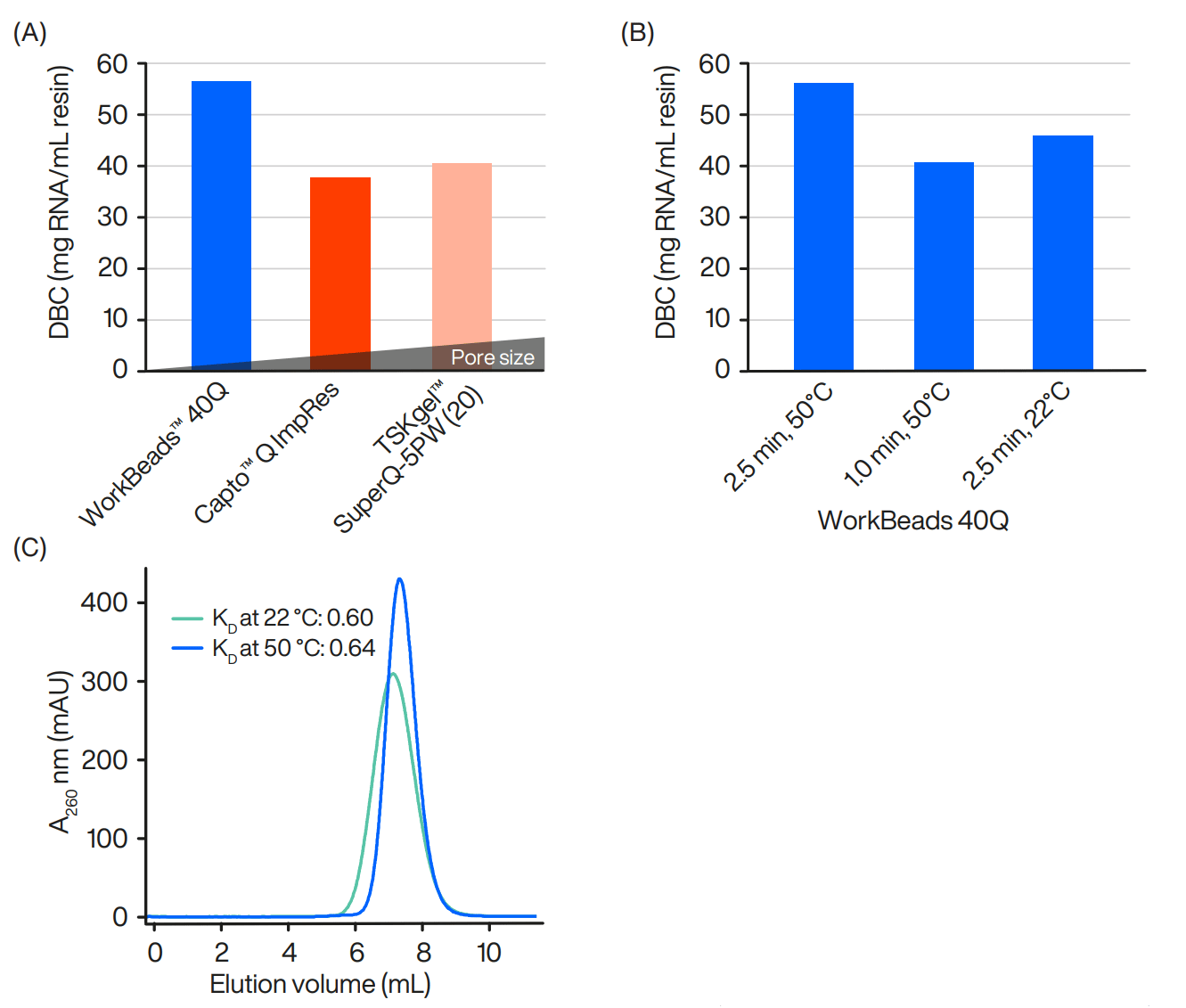
Figure 1: DBC comparison on different AIEX resins (A), using 20 mM NaPO4, 7% acetonitrile, pH 7 at 50 ºC, residence time 2.5 minutes (120 cm/h). Column size 6.6 × 50 mm (1.7 mL). DBC on WorkBeads 40Q (B) using 20 mM NaPO4, 7% acetonitrile, pH 7 at different residence times and temperatures. KD analyses (C) at 22 ºC (green line) or 50 ºC (blue line) of RNA in the presence of PBS, 7% ACN buffer, pH 7.4 on a column (10 x 100 mm) packed with the base matrix used for production of WorkBeads 40Q.
KD analyses – RNA molecules behave as very big molecules.
Heat increased pore volume usage – higher DBC
Purity and yield
12.5 mg RNA feed (79.5% purity) was loaded onto WorkBeads 40Q (13% of DBC) under the optimized conditions described earlier. The main peak obtained was collected in 1-mL fractions. Each individual fraction was analysed for yield and purity on a DNAPac™ PA200 analytical IEX column (Thermo Fisher Scientific).
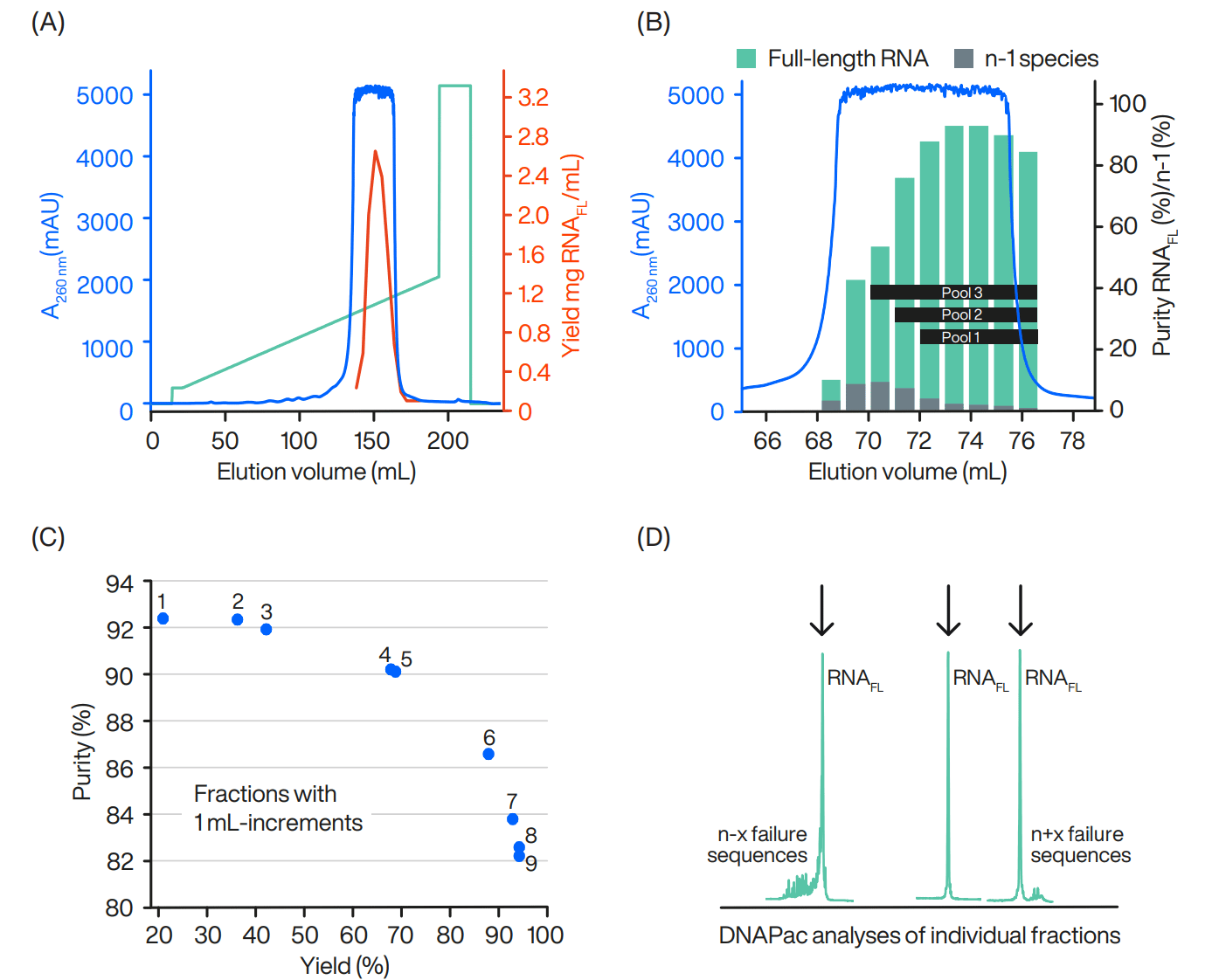
Figure 2: Eluted RNA peak after loading 12.5 mg of crude RNA (A). Red line represents full-length yield measured in each fraction. Purity of full-length RNA (green bars) was measured in individual fractions (B). Gray bars represent n-1 species. Pools of collected fractions are visualized in the chromatogram. The inverse correlation between purity and yield is shown for fractions with 1 mL-increments (C). The numbers next to the dots in the plot represent elution volume in mL of the main peak in Figure 2B. Purity analyses shown for selected fractions (D), illustrating where the failure sequences (that should be removed) end up in the eluted peak.

Table 1: Effect on yield and purity with different pools.
Summary results
• WorkBeads 40Q – highest DBC
• Elevated temperature – increased DBC, increased pore accessibility and homogenous solution
• Very high flow rates – reduced DBC
Conclusions
We have demonstrated which parameters are important in influencing loading capacities for RNA, such as residence time (flow rate), temperature, and in particular, resin type. Purification of the RNA showed good separation of full-length RNA from failure sequences with an inverse correlation between purity and yield.
Bio-Works and WorkBeads are trademarks of Bio-Works Technologies. Capto is a trademark of Cytiva. TSKgel is a trademark of Tosoh Corporation. DNAPac is a trademark of Thermo Fisher. All third-party trademarks are the property of their respective owners.
© Bio-Works 2022. All goods and services are sold subject to Bio-Works terms and conditions of sale. Contact your local Bio-Works representative for the most current information.
Bio-Works, Virdings allé 18, 754 50 Uppsala, Sweden. For local office contact information, visit bio-works.com/contact.