Introduction
The purity requirements are high for monoclonal antibodies (mAbs). Using protein A chromatography resins result in mAbs with high yield and purity in a single step and is therefore the method of choice. Since the cell supernatant containing the expressed mAb often is loaded directly onto the protein A column without any significant pre-purification, it will result in extensive bioburden on the resin. To increase the lifetime of the protein A resin and to improve the final purity of the mAb we have included an upstream multimodal AIEX-pre-treatment column in our process.
WorkBeads™ 40 TREN
WorkBeads™ 40 TREN is a multimodal anion exchange chromatography (AIEX) resin with a ligand (Tris(2-aminoethyl)amine, TAEA) that is positively charged below pH 9. The TAEA-ligand is capable of multiple modes of interactions, such as ionic and hydrophobic interactions. The resin has been demonstrated to be effective in removal of a diverse set of host cell impurities and is therefore an excellent choice as a pre-treatment column when purifying mAbs.
Experimental setup
To study the potential positive effects of WorkBeads 40 TREN as a pre-treatment column, a prepacked BabyBio™ TREN 5 ml column was placed upstream of the protein A column (WorkBeads affimAb). See Figure 1 for the hardware setup.
Figure 1. Hardware setup of the mAb purification process. (A) Flow chart during sample load. (B) Flow chart during the elution of mAbs.
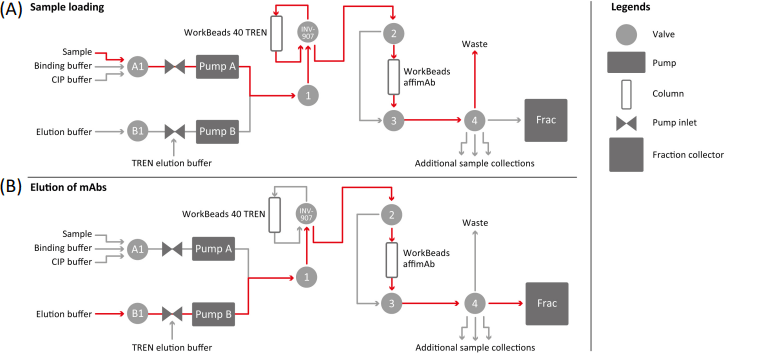
To validate the advantage of WorkBeads 40 TREN as a pre-treatment column, a full experiment setup was designed including two protein A resins, WorkBeads affimAb and MabSelect SuRe™ (GE Healthcare), with two different sample loading conditions (low and high sample load). Thus, a total of eight mAb purifications were performed. The host cell proteins (HCP) and host cell DNA (HCD) were analysed at different stages of the purification.
Results
An example of a mAb purification included in the experimental setup is shown in Figure 2.
Figure 2. A mAb purification where 100 ml clarified CHO cell supernatant is loaded onto WorkBeads 40 TREN and WorkBeads affimAb connected in series. Impurities in the sample will bind to WorkBeads 40 TREN.

SDS-PAGE analysis of the purifications is shown in Figure 3. Analyses of flow-throughs with or without WorkBeads 40 TREN demonstrated a 95% removal of HCP and 99% removal of HCD due to the addition of WorkBeads 40 TREN (data not shown).
Figure 3. SDS-PAGE analysis of fractions from the eight runs with combinations of protein A resins with or without pre-treatment using WorkBeads 40 TREN. A: WorkBeads affimAb, M: MabSelect SuRe, T: WorkBeads 40 TREN. FT: flow through and E: eluted mAb. (A) 20 ml load WorkBeads 40 TREN and (B) 100 ml load.

SDS-PAGE analysis of the purifications showed a significant reduction of host cell impurities in the mAb eluates when WorkBeads 40 TREN was included. A major part of HCP and HCD were adsorbed to WorkBeads 40 TREN with no loss of mAb recovery, i.e., no binding of mAbs to the pre-treatment resin.
Table 1. Analysis of collected mAb eluates from eight runs with combinations of protein A resins with or without pre-treatment using WorkBeads 40 TREN.
| Resins |
Sample load (ml) |
HCP (ppm) |
HCD (ppm) |
| WorkBeads affimAb |
20 |
667 |
4 |
| WorkBeads 40 TREN + WorkBeads affimAb |
20 |
224 |
1 |
| MabSelect SuRe |
20 |
8016 |
114 |
| WorkBeads 40 TREN + MabSelect SuRe |
20 |
347 |
1 |
| WorkBeads affimAb |
100 |
2009 |
12 |
| WorkBeads 40 TREN + WorkBeads affimAb |
100 |
1404 |
10 |
| MabSelect SuRe |
100 |
10938 |
80 |
| WorkBeads 40 TREN + MabSelect SuRe |
100 |
7331 |
69 |
The HCD and HCP analyses showed higher purity of the mAb eluates when
WorkBeads 40 TREN was included. It additionally demonstrated that
WorkBeads affimAb yields mAbs with higher purity and high yield compared to
the market leading resin (Table 1, and Figure 4).
Figure 4. HCP (A) and HCD (B) analyses of the collected mAb eluates. A: WorkBeads affimAb,
M: MabSelect SuRe and T: WorkBeads 40 TREN.

Conclusion
WorkBeads 40 TREN resin was shown to remove up to 95% of the host cell proteins and 99% of the host cell DNA from the feed loaded onto the protein A columns. WorkBeads 40 TREN effectively reduces the bioburden on the protein A column which significantly extends its lifetime. Adding WorkBeads 40 TREN to the process will also contribute to a higher purity of the mAb.