Introduction
Immobilized Metal Affinity Chromatography (IMAC) is the commonest technique for purification of His-tagged proteins. Tagging proteins enables the use of affinity chromatography for purification and results in very efficient purification. In a single step the purity can be as high as ≈ 80%. This purity is sufficient for several applications but in many cases higher purities are required, as well as higher yields, and then comes the challenge of optimizing the IMAC purification setup. Below are some optimization hints and tips.
PROS and CONS for different immobilized metal ion resins
Nickel (Ni2+)
- Most used metal ion and many scientific references available
- EDTA/DTT resistant resins available, e.g. WorkBeads™ NiMAC
- High yields usually obtained
- First choice when time is limited for doing a screening
- May give slightly lower purity compared to others
- Not environmentally friendly
Cobalt (Co2+)
- Gives generally high purity
- Often the second option to Ni2+
- May give slightly lower yield compared to others
- Not environmentally friendly
Zinc (Zn2+)
- Best choice for bioprocess scale
- Least toxic → good for purification of drugs for human use
- Environmentally friendly
- Often forgotten when testing
Copper (Cu2+)
- Could be tested when other metal ions don’t give good results
- Environmentally OK
- Often forgotten when testing
Different metal-ligand combinations give different selectivities
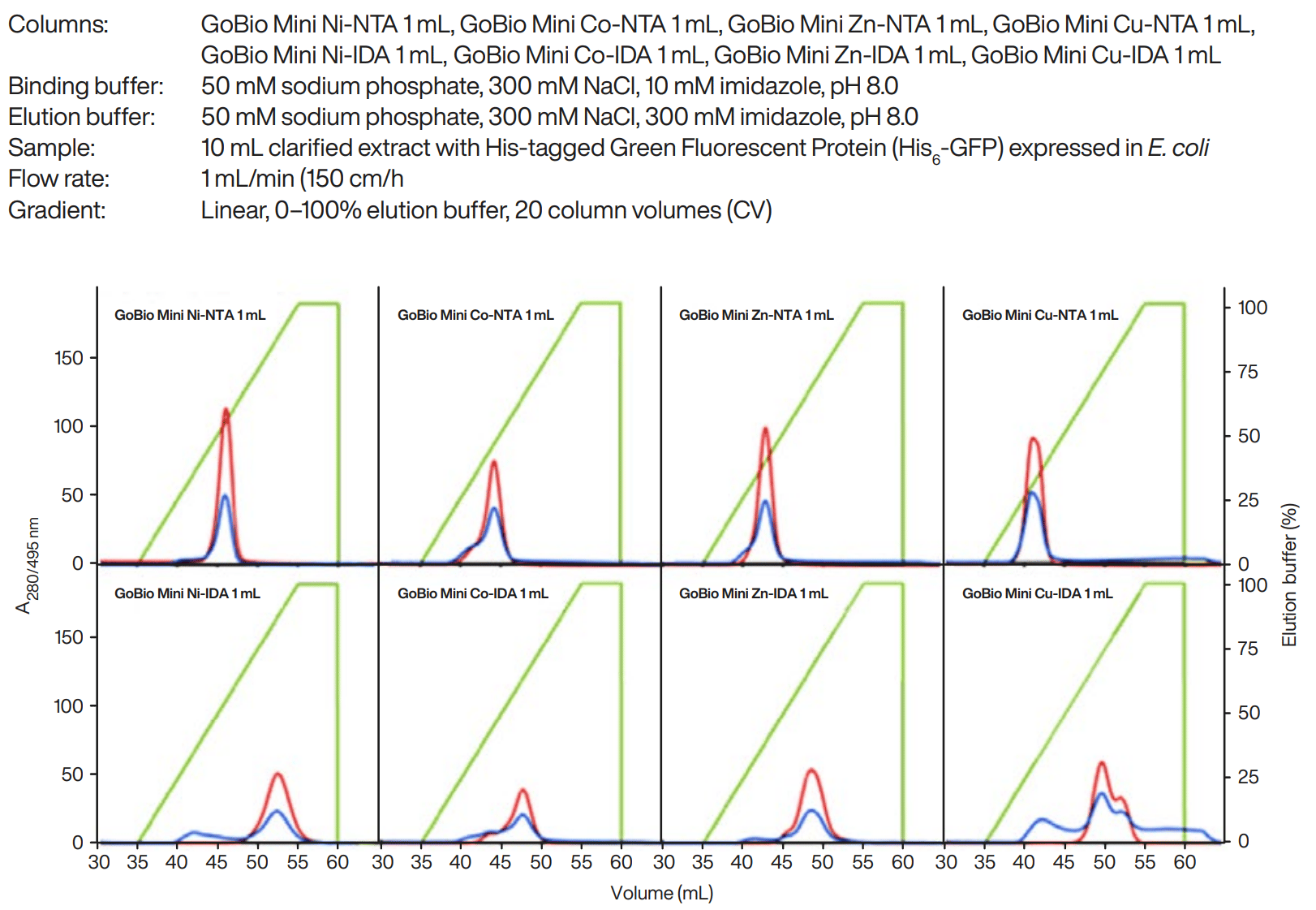
Figure 1. Comparison of purifications of His6-GFP expressed in E. coli on GoBio™ Mini NTA and GoBio Mini IDA 1 mL charged with Ni2+, Co2+, Cu2+ and Zn2+ at 1 mL/min (150 cm/h). Binding buffer: 50 mM sodium phosphate, 300 mM NaCl, 10 mM imidazole, pH 8.0. Elution buffer: 50 mM sodium phosphate, 300 mM NaCl, 300 mM imidazole, pH 8.0. Blue: absorbance, 280 nm. Green: absorbance, 495 nm (specific for His6-GFP). Red: elution buffer, % (linear gradient 0-100%, 20 CV).
Common problems, hints and tips, what to do?
Low purity
- Optimize binding and wash buffers – increase imidazole concentration
- Use high quality imidazole (should be a white powder)
- Use screening kit to find optimal metal ion (Ni2+, Co2+, Cu2+, Zn2+)
- Optimize length of His-tag to increase binding
- Optimize elution, step vs. linear gradient, increase CV* of gradient
- Change to pH gradient (can be tricky)
- Use NTA ligand for immobilization of metal ions
- Add a second purification step, e.g. SEC or IEX
* Column volume
Low yield – Inefficient elution
-
Too tight binding to resin
- Optimize metal ion (Ni2+, Co2+, Cu2+, Zn2+)
- Use IDA ligand for immobilization of metal ions
- Decrease length of His-tag
-
Elution buffer composition not optimal
- Optimize imidazole concentration
- Change to pH gradient
-
Precipitation
- Decrease amount of sample loaded on column
- Decrease concentration of imidazole in buffers
- Change to pH gradient

Low yield – Poor binding
-
Target protein is degraded
- Work fast! Don’t start and then go for a coffee break
- Work at lower temperature, e.g. +4–8°C
- Add proteases. Remember, what you add has to be removed!
-
Tag doesn’t bind tight enough
- Optimize metal ion (Ni2+, Co2+, Cu2+, Zn2+)
- Increase length of the His-tag
-
Tag is not exposed for binding
- Do purification under denaturing conditions
- Move tag to N-/C-terminal or change to another tag (GST, Strep)
-
Substances in cell culture medium interfere with binding
- Use Ni-resin resistant to EDTA/DTT
- Desalt before using IMAC
-
Buffer composition not optimal
- Decrease amount of imidazole in binding/wash buffers
- EDTA/DTT in buffers stripping metal ions from resin
- Screen for optimal buffer composition
-
The kinetics between ligand and tag is slow
- Decrease flow rate to residence time > 6 min
- Use NTA ligand for immobilization of metal ions
...but if there is no time for resin screening?
- Use Ni-NTA resin, if yield is most important
- Use Co-NTA resin, if purity is most important
- Use WorkBeads NiMAC resin if there is EDTA/DTT in cell culture media/buffers or expression in eukaryotic cells