Introduction
A protein A ligand designed to give high alkaline stability was immobilized on WorkBeads™ basematrix. The combination of ligand concentration and resin properties was optimized for high binding capacity for monoclonal antibodies (mAbs) and stability towards alkaline cleaning-in-place (CIP) conditions.
The resin, WorkBeads affimAb, is under development and designed to give high binding capacity also at high sample application flow rates, i.e., high dynamic binding capacity over a broad range of flow rates. The functionality is compared with similar products from other suppliers. The optimized ligand sequence and stabile covalent immobilization allowed for efficient CIP using 0.5 M NaOH.
High dynamic binding capacity
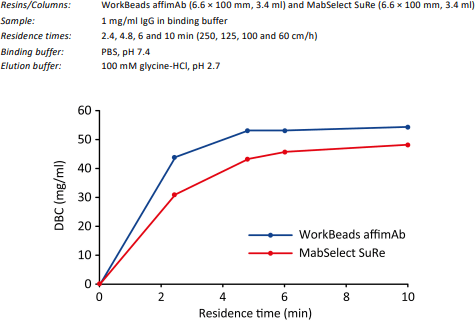
WorkBeads affimAb is designed to give a dynamic binding capacity (DBC) of typically more than 40 mg IgG/ml resin under standard binding conditions. DBC at different flow rates (residence time, RT) determined by frontal analysis at 10% breakthrough for WorkBeads affimAb were compared to MabSelect SuRe™, see Figure 1.
Figure 1. Dynamic binding capacity for human polyclonal IgG determined at different flow rates (residence times) by frontal analysis.
Preserved binding capacity over many cleaning-in-place cycles
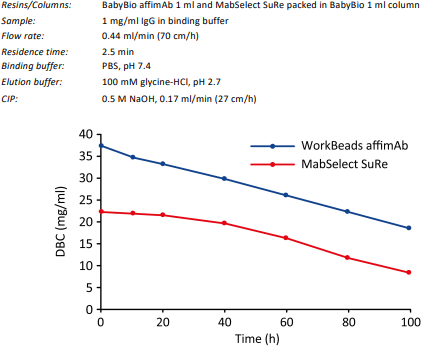
The dynamic binding capacity after typical CIP treatment was investigated using continuous incubation with 0.5 M NaOH for 100 h at 0.17 ml/min. DBC was determined regularly, see Figure 2. Theoretically, 100 h of treatment corresponds to 400 CIP cycles at 15 min.
The study was performed in BabyBio™ affimAb 1 ml column and MabSelect SuRe packed in BabyBio 1 ml column. The lower DBC values in this study compared to values in Figure 1 are caused by the shorter bed height (28 mm) which results in lower binding capacity compared to columns with 100 mm bed height at the same residence time.
The design combination of the alkaline stable recombinant protein A with an optimized basematrix results in both high dynamic binding capacity and the possibility for efficient cleaning-in-place with 0.5 M NaOH.
Figure 2. Comparison of dynamic binding capacity of WorkBeads affimAb and MabSelect SuRe after in total 100 h treatment with 0.5 M NaOH.
Purification of a monoclonal antibody
An example of using WorkBeads affimAb for a purification of a monoclonal antibody expressed in CHO cells is shown in Figure 3A. Purity analysis was analyzed by SDS-PAGE, see Figure 3B. A comparison with MabSelect SuRe is included.
Figure 3. Purification profile of a monoclonal antibody from clarified CHO cell supernatant. Eluted pool is shown in red (A). Purity check on SDS-PAGE (B).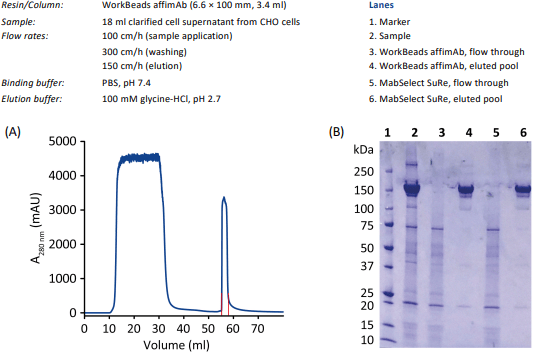
Conclusions
The WorkBeads affimAb resin is designed for high dynamic binding capacity and stabile binding capacity over extended alkaline treatment with 0.5 M NaOH. The binding capacity remains high also at higher flow rates. The purity obtained of CHO-cell expressed monoclonal antibody on WorkBeads affimAb was equal to MabSelect SuRe under identical conditions.